Tackling Meningitis in Africa with MenAfriVac
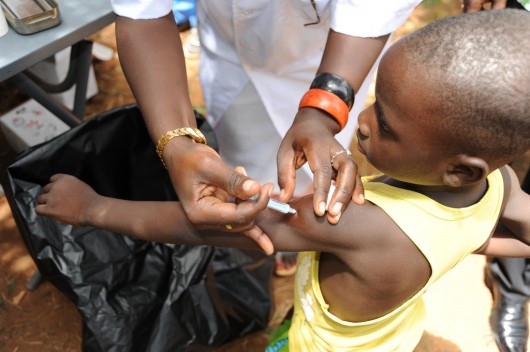 On Feb. 22, 2016, vaccine experts from all over the world convened in Ethiopia with leaders from the 26 African “meningitis belt” countries to celebrate the success achieved by MenAfriVac, a vaccine created for use in Africa.
On Feb. 22, 2016, vaccine experts from all over the world convened in Ethiopia with leaders from the 26 African “meningitis belt” countries to celebrate the success achieved by MenAfriVac, a vaccine created for use in Africa.
The vaccine was developed specifically for Africa and targets meningococcal A meningitis, a bacterial infection of the thin lining surrounding the brain and spinal cord. Meningitis is a highly-feared disease due to its capacity to kill its host within hours. Survivors often experience permanent hearing loss, paralysis or even mental retardation.
“We have achieved something truly historic with MenAfriVac®—creating an affordable, effective, tailor-made vaccine for Africa,” said Steve Davis, president and CEO of PATH, a nonprofit global health organization.
According to PATH, more than 90 percent of meningitis epidemics in Africa attacked mostly infants, children and young adults. To zero in on this specific cause of meningitis, PATH partnered with the Meningitis Vaccine Project and the World Health Organization (WHO).
In the five years that MenAfriVac has been in effect, 235 million children and adults have been vaccinated. From 250,000 cases during an epidemic from 1996 to 1997, to only 80 confirmed cases in 2015, the vaccine has effectively protected millions of people.
However, a resurgence is possible within 15 years if an immunization program is not implemented permanently. Several countries applied for funding to begin implementing MenAfriVac into their national childhood immunization programs. Gavi, a global health partnership that focuses on vaccines, has spent $367 million campaigning and stockpiling the vaccine since 2008 to support these countries.
“Meningitis A was a scourge across Africa’s meningitis belt for generations but today we can be proud that a safe, effective meningitis vaccine is protecting hundreds of millions of people from death and disability,” said Dr. Seth Berkley, Gavi CEO. “But we must not be complacent. It is critical that at-risk countries begin introducing this vaccine into their routine schedules and ensuring every child is reached and protected.”
This achievement could not have been possible without the vital partnerships that contributed to the development of the vaccine. U.S. agencies financially supported MenAfriVac, provided technical expertise and participated in clinical studies of the vaccine.
Continued partnerships could lead to solutions for other diseases around the world and have a positive impact on global health.
– Emily Ednoff
